Biblioteca Istituto Zooprofilattico Sperimentale dell'Umbria e delle Marche
Sanità Pubblica Veterinaria: Numero 100, Febbraio 2017 [http://www.spvet.it/] ISSN 1592-1581
Documento reperibile all'indirizzo: http://spvet.it/indice-spv.html#651
Infectious agents identified in ticks collected in Umbria Region (Italy)
Agenti infettivi identificati in zecche raccolte nella Regione Umbria
Silva Costarelli, Jacopo Zema, Angela Caporali, Caterina Mariotti, Carmen Panzieri, Carmen Iscaro, Cristina Canonico, Monica Giammarioli, Stefano Gavaudan
Istituto Zooprofilattico Sperimentale dell'Umbria e delle Marche. Perugia (Italy)
Abstract. Ticks can transmit viruses, bacteria and protozoa playing an important role in the epidemiology of tick borne diseases (TBDs). Because the ticks are reliable indicators for the existence of pathogens in a specific area, it is recommended to periodically screen animals and vectors for pathogen carriage. Umbria is a central region of Italy where the environmental features (macro and microclimate, deciduous forests, presence of a consistent population of wild boar and roe deer) offer ideal conditions for living of different species of ticks. The aim of the present study was to get data on the most commune tick-hosted pathogens in Umbria. Ticks were collected from environment and/or from animals skin. A total of 505 ticks were collected. PCR tests and DNA sequences revealed several pathogens. Rickettsia spp. was the most detected. The second infectious agent was Anaplasma phagocytophilum. In order to verify the possible spread of TBEv in domestic and wild species of the region, 390 sera from sheep and 211 sera from different species of wild animals were tested. Serological data evidenced the absence of TBE infection in tested animals. Negative the results for TBE virus in all the Ixodes spp. ticks
Riassunto.
I casi di malattie trasmesse da vettori sono aumentati notevolmente negli ultimi anni in Europa ed in Italia e, parallelamente, è cresciuta la sensibilità dei cittadini rispetto a questa problematica. Sono sempre più frequenti, ad esempio, coloro che, interessati da morsi di zecca, ricorrono alle prestazioni dei pronto soccorsi per sottoporsi a specifici protocolli di profilassi antimicrobica e, talora, farsi rimuovere il parassita.
Le caratteristiche ambientali, ecologiche e climatiche dell'Umbria creano condizioni favorevoli ad una significativa presenza di zecche. Ciò nonostante, nel nostro territorio, i rilievi epidemiologici sulla diffusione di tali parassiti e sul loro possibile ruolo nella trasmissione di agenti infettivi all'uomo, sono piuttosto limitati.
Eppure il crescente proliferare di popolazioni ospiti naturali, la longevità delle zecche, la loro elevata capacità riproduttiva, la scarsità di nemici naturali, la resistenza ai pesticidi ed alle condizioni ambientali sfavorevoli, potrebbero motivare un aumento della loro diffusione e, di conseguenza, del rischio di trasmissione di zoonosi (Tick Borne Diseases - TBD).
Scopo del presente studio è stato quello di aggiornare i dati sulle specie di zecca presenti in Umbria e sui più comuni patogeni che possono ospitare. I parassiti sono stati raccolti dall'ambiente e/o direttamente dalla cute di animali parassitati. Nell'ambito di una stagione primaverile sono state raccolte 505 zecche sottoposte a test di PCR e successivo sequenziamento. I risultati hanno fatto emergere la diffusione di diverse specie di Rickettsia e la presenza, più contenuta, di Anaplasma phagocytophilum, Babesia spp., Francisella spp. Negativi, al contrario, i risultati per il TBE-virus nelle zecche appartenenti al genere Ixodes e gli esiti di un monitoraggio sierologico per TBE effettuato in specie domestiche e selvatiche della regione.
Introduction
Ticks are arthropods belonging to the order of Aracnida. They are haematophagous obligate and parasitize every class of vertebrate.
They require a blood meal during at least one life stage. Due to this characteristic, ticks can transmit a wide range of infectious agents such as viruses, bacteria and protozoa, to different hosts. As arthropod vectors of disease agents they are considered second only to mosquitoes (Parola e Raoult, 2001) and playing an important role in the epidemiology of tick borne diseases (TBDs) of humans and animals.
The impact of ticks on human public health is due to the fact that many important zoonotic TBDs are worldwide increased, gaining more attention from physicians and veterinarians. Hence it is important a better knowledge of these diseases.
Since ticks are reliable indicators for the existence of pathogens in a specific area, it is recommended to periodically screen animals and vectors for pathogen carriage.
Generally, the risk of a TBD is dependent on prevalence of infectious vectors and on the opportunity of their contact with susceptible hosts.
Therefore their distribution determines the epidemiology of vector borne infections in a territory. In central Italy the macro and micro-climate, the vegetation features characterized by deciduous forests, the consistent presence of some wild species (wild boar, roe deer) offer ideal conditions of living for different species of ticks.
Because the first step towards a TBDs surveillance plan is the knowledge of the pathogens occurring in a given area and their prevalence in vectors and hosts (Capelli 2012), the objectives of the study have been:
- to collect and to identify ticks from environment or from animals of the territory
- to detect the presence of emerging pathogens in all the collected ticks
- to get data on TBE infection in wildlife and domestic animals (sheep) in our region
Material and methods
Tick collection
The study has been carried out in Umbria region (central Italy) during the spring season. Ticks were collected from either the vegetation by dragging either directly from the skin of the animals in the necropsy facility of our Institute.
Furthermore, several ticks were picked up from living animals during field and farm visits. Once collected, ticks were kept chilled in a RNA later solution. They were counted and identified by species and life stage according to the keys by Manilla (Manilla 1988), than grouped in engorged or non engorged specimens.
DNA and RNA were extracted with "AllPrep DNA/RNA mini kit" (Qiagen), according to the manufacturer's instructions. The nucleic acids were extracted from single adult ticks, or pools: 70 ticks (the adult ones or the more engorged specimens) were subjected to individual extraction, the others were extracted in pool for a total of 84 pools. One pool consisted of 5-6 non engorged or young specimens, in the case the same species was collected from the same host/area. Several PCR methods were used for the following pathogens detection: TBE Flavivirus (Schwaiger e Cassinotti 2003), Rickettsia spp. (Raox et al., 1997), Anaplasma phagocytophilum (Massung e Slater 2003), Borrelia spp. (Skotarczak 2002), Babesia spp. (Hilpertshauser 2006) and Coxiella burnetii (Berri et al., 2000; Magnino 2009). To ensure the effectiveness of the nucleic acid extraction, a real time PCR targeting the 16S rRNA was applied (Schwaiger e Cassinotti 2003).
Serologic findings
Since sheeps and goats are considered as ideal sentinels for TBE (Klaus et al., 2012), to get data on TBE infection in domestic animals and wildlife, blood sera of 390 sheep, randomly collected from 130 farms of Umbria region, were analyzed. The number of sheep sera to determine seroprevalence was calculated assuming a quite large population (50% prevalence, 5% accuracy, 95% confidence level). To detect antibodies against TBE virus, a competitive ELISA kit (EIA TBEv Ig, Test-Line Clinical Diagnostics, Czech Republic) was used. In addition, 211 sera collected from 141 wild boars, 66 roe deers and 4 fallow deers, were also analyzed.
Results
A total of 505 ticks were collected. Overall six species were identified belonging to four genera: 207 Rhipicephalus sanguineus (40,9%), 105 Hyalomma marginatum (20,8%), 78 Ixodes ricinus (15,4%), 68 Dermacentor marginatus (13,5%) 46 Rhipicephalus bursa (9,1%), 1 Ixodes hexagonus (0,2%) (Figure. 1: "Number and species of collected ticks"). Since the most specimens were collected from animals and not by dragging, the adult ticks predominated over immature ones (Figure. 2: "Number and development stage of collected ticks"). Among the Ixodes ricinus, 53 were adult ticks (68%), 21 nymphs (27%) and 4 larvae (5%), (Figure. 3: "Number and development stage of Ixodes ricinus collected ticks").
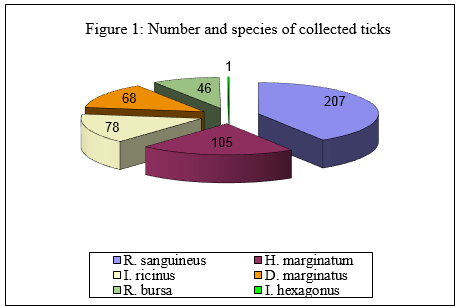
Immagine 1. Numero e specie di zecche campionate
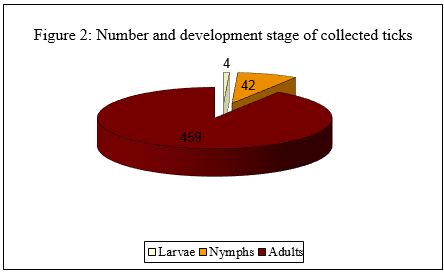
Immagine 2. Numero e stadio di sviluppo delle zecche campionate
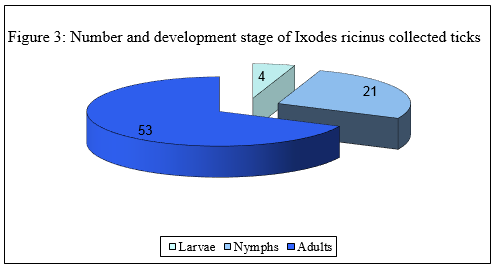
Immagine 3. Numero e stadio di sviluppo di zecche
Iodes ricinus campionate
PCR tests and DNA sequences detected several pathogens in thirty-eight single ticks (54,3%) and in fourty-five pools (53,6%), as listed in the table 1 ("Number and % of positive single ticks or pools, for each pathogen").
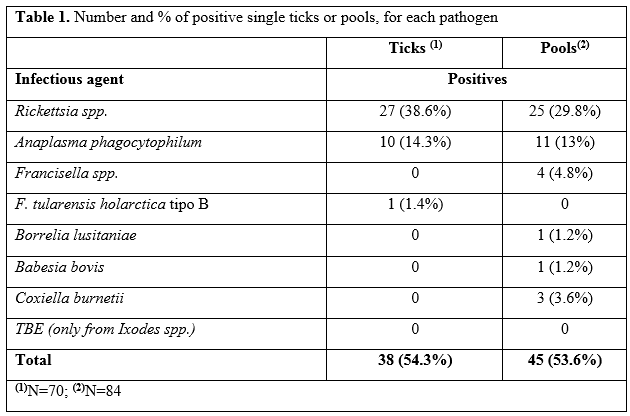
Tabella 1. Numero e percentuale di singole o gruppi di zecche positive per ogni patogeno
Among the pathogens, Rickettsia spp. was isolated in 38,6% of the individually extracted ticks and in 29,8% of the pools.
The second infectious agent most isolated was Anaplasma phagocytophilum. The infectious agents isolated from ticks species and their hosts, or by dragging, are reassumed in table 2 ("Host animals species, number of collection sites, ticks species and their number").
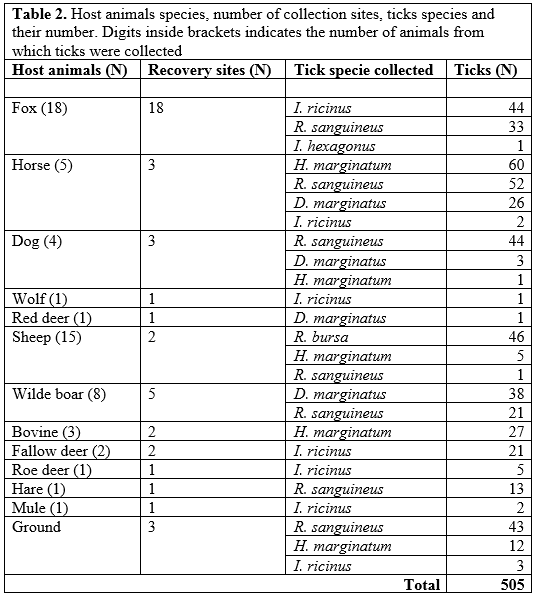
Tabella 2: Specie di animali ospiti, numero di siti di raccolta, specie di zecche e loro numero. Le cifre fra parentesi indicano il numero di animali dai quali sono state raccolte le zecche
The PCR tests resulted negative for TBE virus in all the Ixodes spp. ticks.
The seroprevalence for TBE virus was estimated at 0,8% (I.C.95%: 0,2% - 2,4%) in sheep population. All the wild animals resulted negative.
Discussion and conclusion
As far we know, this work represents the first survey on the presence of infectious agents in tick specimens performed in Umbria region. Interesting, the animal hosts from which ticks were collected, came from many different areas of the region (Figure 4). This fact allowed us to have a wide view of the regional reality.
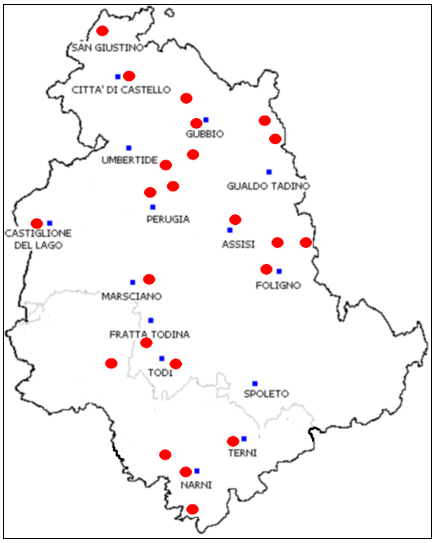
Figure 4. Red points: Origin sites of host animals or dragging activities
Figura 4. Punti rossi: siti di proventienza di animali ospiti
The most interesting result is the detection of many Rickettsia species, showing their diffusion in the territory.
Tick-borne rickettsioses are among the oldest known vector-borne zoonotic diseases (Parola et al., 2005) but, till few years ago, it was thought that the only tick-borne rickettsiosis in Europe was due to Rickettsia conorii.
However, by the use of new sequence-based molecular techniques, in the last years new emerging rickettsia species have been documented. Current opinion is that human rickettsial infections have too often gone unrecognized and undiagnosed (Lo et al., 2004; Beninati 2002).
Our research-work seems to confirm these data because several Rickettsia species were isolated in engorged and not engorged ticks (Table 3).
Among the Rickettsiae recognized as human pathogens, we detected R. slovaca, R. aeschlimannii, R. monacensis. and R. helvetica.
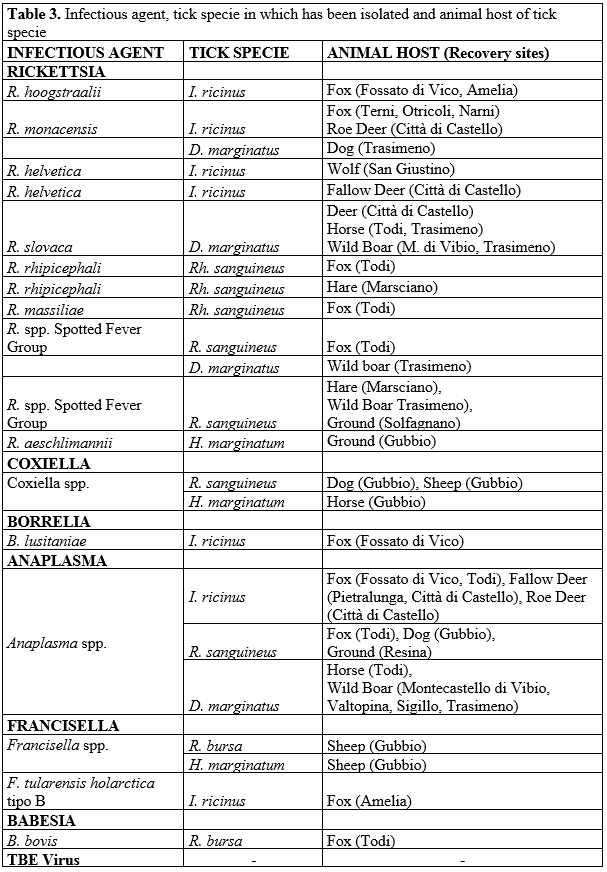
Tabella 3. Agente infettivo, specie di zecca nella quale è stato isolato e animale ospite
Because isolated rickettsiae species are zoonoses agents, their presence could represent and cause public health problems.
R. slovaca was detected in D. marginatus ticks obtained from two wild boars, one red deer and two horses. For many years R. slovaca has been considered a nonpathogenic rickettsia but actually it is recognized as the eziopathogenetic agent of a clinical syndrome named "tibola" (TIck BOrne LymphAdenopathy) or "debonel" (Dermacentor-borne-necrosis-erythema-lymphadenopathy), characterized by fever, chronic fatigue, headache, eschar at the tick bite site, regional painful, lymphadenopathy and, sometimes, rush (Blanco e Oteo 2006).
In central Italy R. slovaca was isolated in 35% of human patients included in a Surveillance System in the Lucca Province and bitten by D. marginatus during the years 2005-2006 (Martello et al., 2009), confirming the close relationship between pathogen and carrier, and the infection risk for humans.
Until some years ago, it was believed R. aeschlimannii as present in tick of northern Africa areas, only. During the last years more and more isolations of R. aeschlimannii were done in several countries of south Europe, such as Croatia (Punda Polic et al., 2002), Spain (Fernandez Soto 2003) and Greece (Papa 2016).
The results of our survey highlight the presence of such a parasite on our territory (Umbria region), too.
In the human, the infection is characterized by eschar at the tick-bite site, fever, maculopapular generalized skin rush. In some cases, because H. marginatum has the feature to prick repeatedly in the same small skin area, multiple eschars can be revealed.
A high percentage of ticks were also positive to A. phagocytophilum, both in individually tested ticks and in not engorged specimens. Human anaplasmosis due to A. phagocytophilum was first reported in Slovenia in 1997 (Petrovec et al., 1997). To date numerous cases have been reported in many European countries (Jin et al., 2012), also in Italy where different studies have been conducted on the presence of A. phagocytophilum in the tick vectors and in the wild and domestic reservoirs. It seems that A. phagocytophilum presence embraces the whole Italian territory from the Alps to the southern and insular regions (De la Fuente et al., 2005; Alberti et al., 2005; Ruscio e Cinco 2003).
In central Italy, it has been reported in equines, dogs and sheep, (Lillini et al., 2006; Beltrame et al., 2006; Di Domenico et al., 2016; Passamonti et al., 2010).
Limited serological surveys and other diagnostic investigations have been carried out to know the tick-borne infection state in livestock of our region. However sporadic positive cases in randomly tested animals (sheep overall) are found and it may indicate that vector-borne pathogens could affect herds or flocks more than it was supposed. Based on our first results it should be encouraged further investigations for a better understanding prevalence and incidence of rickettsial infections within the farms. Flocks and herds should be tested and a tick survey should be performed in the proximity of the farms with the aim to identify some possible risk-areas.
Borrelia burgdorferi has not been detected in ticks and no Lyme disease cases are officially registered in Umbria by the Health Regional Office.
B. lusitaniae was isolated in an engorged I. ricinus collected from the skin of a fox. B. lusitaniae is one of the known Borrelia genospecies frequently found in Europe and it is identified as the predominant Borrelia genospecie in central Regions such as Tuscany, Marche and Lazio (Bertolotti et al., 2006; Amore et al., 2007; Pascucci e Cammà 2010; Veronesi et al., 2012).
Seroprevalence data are not available in Umbria Region, either regarding animal hosts or humans.
Isolation of Francisella tularensis holarctica type B in a specimen of I. ricinus collected by a fox confirms the presence of this pathogen in Umbria territory. The last human case of tularemia in the Region has been officially notified to the Health Ministry in 2006. Since the notification is not always easy to update by physicians, it should be to expect a greater spread of the infection. In the near Tuscany region 42 human cases were notified in 2008.
Regarding the TBEv diffusion we know that Italy is considered a country at low risk for this infection which seems to be restricted to some regions of its territory. We worked in a supposed non endemic area because, actually, no human TBE cases are registered in Umbria.
Our results confirm this assumption. The limited values of seroprevalence (0,8%) estimated in the sheep population and the negative results in wildlife suggest that, at present, there is no virus circulation in the region.
Unfortunately our samples were limited at few hundreds of ticks and sera and do not to allow a significative epidemiological conclusions on the true TBEv diffusion in this central part of Italy. Since TBE is a zoonosis few results about virus diffusion in vectors or about immuno-competence in domestic/wild species can help clarifying the risk to humans and to setting up adequate surveillance systems.
Recently because the increasing interest of human involved population, human doctors and researchers it was a positive pushing to clarify dinamics and epidemiological scenarios.
In fact, not only cases of vector-borne diseases, in Europe and in Italy, have considerably increased in recent years but, in parallel, also the citizens' sensitivity to these issues has grown over the time.
More and more people, affected by tick bites, had received emergency care to get remove the carrier (tick) from the injection (bite) site and had been undergone to specific antimicrobial prophylaxis protocols.
Always more often hospitalizations of patients with flu-like symptoms, skin rash or encephalitis are recorded. Despite advances in diagnostic techniques, every year a large percentage of these patients are still dismissed with a diagnosis of "unknown origin."
This suggests that there are, even today, large lacks in the territorial knowledge of the epidemiology of these infections that results in the absence of adequate diagnostic protocols.
Bibliography
Alberti A., Zobba R., Chessa B., Addis M. F., Sparagano O., Pinna Parpaglia M. L., Cubeddu T., Pintori G., Pittau M., (2005). Equine and canine Anaplasma phagocitophilum strains isolated on the Island of Sardinia (Italy) are phylogenetically related to phatogenic strains from United States. Applied and Environmental Microbiology. 71 (10), 6418-6422.
Amore G., Tomassone L., Grego E., Ragagli C., Bertolotti L., Nebbia P., Rosati S., Mannelli A. (2007). Borrelia lusitaniae in Immature Ixodes ricinus (Acari: Ixodidae) feeding on common wall lizards in Tuscany, Central Italy. Journal of Medical Entomology. 44, (2): 303-307.
Beltrame, A., Ruscio, M., Arzese, A., Rorato, G., Negri, C., Londero, A., Crapis, M.,Scudeller, L., Viale, P., (2006). Human granulocytic anaplasmosis in Northeastern Italy. Annals of the New York Academy of Sciences. 1078, 106-109.
Beninati T., Lo N., Noda H., Esposito F., Rizzoli A., Favia G., and Genchi C. (2002). First detection of spotted fever group Rickettsiae in Ixodes ricinus from Italy. Emerging Infectious Diseases journal. 8. (9): 983-986.
Berri M., Laroucau K., Rodolakis A. (2000). The detection of Coxiella burnetii from ovine genital swabs, milk and fecal samples by the use of a single touchdown polymerase chain reaction. Veterinary Microbiology. 72, (3-4): 285-293.
Bertolotti L., Tomassone L., Tramuta C., Grego E., Amore G., Ambrogi C., Nebbia P., Mannelli A. (2006). Borrelia lusitaniae and Spotted Fever Group Rickettsiae in Ixodes ricinus (Acari: Ixodidae) in Tuscany, Central Italy. Journal of Medical Entomology. 43, (2): 159-165.
Blanco J. R. and Oteo J. A. (2006). Rickettsiosis in Europe. Annals of the New York Academy of Sciences. 1078: 26-33.
Capelli G., Ravagnan S., Montarsi F., Ciocchetta S., Cazzin S., Porcellato E., Babiker A. M., Cassini R., Salviato A., Cattoli G., Otranto D. (2012). Occurrence and identification of risk areas of Ixodes ricinus-borne pathogens: a cost-effectiveness analysis in north-eastern Italy. Parasites & Vectors, 5:61.
De la Fuente, J., Torina, A., Naranjo, V., Caracappa, S., Di Marco, V., Alongi, A., Russo,M., Maggio, A.R., Kocan, K.M., (2005). Infection with Anaplasma phagocytophilumin a seronegative patient in Sicily, Italy: case report. Annals of Clinical Microbiology and Antimicrobials. 4, 15.
Di Domenico M., Pascucci I., Curini V., Cocco A., Dall'Acqua F., Pompilii C., Cammà C. (2016). Detection of Anaplasma phagocytophilum genotypes that are potentially virulent for human in wild ruminants and Ixodes ricinus in Central Italy. Ticks and tick borne diseases 7, (5): 782-787.
Fernandez-Soto P. (16), Encinas-Grandes A., Perez-Sanches R., (2003). Rickettsia aeschlimannii in Spain: molecular evidence in Hyalomma marginatum and five other tick species that feed on humans. Emerging Infectious Diseases journal. 9: 889-890.
Hilpertshauser H., Deplazes P., Schnyder M., Gern L., Mathis A. (2006). Babesia spp. identified by PCR in ticks collected from domestic and wild ruminants in southern Switzerland. Applied and Environmental Microbiology. (72): 10, 6503-6507.
Jin, H., Wei, F., Liu, Q., Qian, J., (2012). Epidemiology and control of humangranulocytic anaplasmosis: a systematic review. Vector Borne Zoonotic Dis. 12,269-274.
Klaus, C., Beer, M., Saier, R., Schau, U., Moog, U., Hoffmann, B., Diller, R., Süss, J. (2012). Goats and sheep as sentinels for tick-borne encephalitis (TBE) virus - Epidemiological studies in areas endemic and non-endemic for TBE virus in Germany. Ticks and Tick-borne Dis. 3, 27-37.
Lillini E., Macrì G., Proietti G., Scarpulla M. (2006). New findings on anaplasmosis caused by infection with Anaplasma phagocytophilum. Annals of the New York Academy of Sciences. 1081), pp. 360-37.
Lo N., Beninati T., Sacchi L., Genchi C., Bandi C. (2004). Rickettsiosi emergenti. Parassitologia 46: 123-126.
Magnino S., Vicari N., Boldini M., Rosignoli C., Nigrelli A., Andreoli G., Pajoro M., Fabbi M. (2009). Rilevamento di Coxiella burnetii nel latte di massa di alcune aziende bovine lombarde. Large Animal Review; 15: 3-6.
Manilla G. (1988). Fauna d'Italia. Acari Ixodida. Bologna, Italia, Edizioni Calderini.
Martello E., Bisanzio D., Bertolotti L., Tomassone L., Mannelli A., Selmi M. (2009). TIBOLA e Rickettsia slovaca in Lucca, Toscana, Italia. Atti del V Workshop Nazionale di Epidemiologia Veterinaria, ISS, Roma, pg. 70.
Massung R. F., Slater K.G. (2003). Comparison of PCR assay for detection of the agent of human granulocytic ehrlichiosis, Anaplasma phagocytophilum. Journal of Clinical Microbiology. (41): 2, 717-722.
Papa A., Xanthopoulou K., Kotriotsiou T., Papaioakim M., Sotiraki S., Chaligiannis I., Maltezos E.(2016). Rickettsia species in human-parasitizing ticks in Greece. Transactions of the Royal Society of Tropical Medicine and Hygiene. 110 (5): 299-304.
Parola P. and Raoult D. (2001). Ticks and Tickborne Bacterial Diseases in Humans: An Emerging Infectious Threat. Clinical Infectious Diseases, 32, 897-928.
Parola P., Paddock C. D., Raoult D. (2005). Tick-borne Rickettsioses around the world: emerging diseases challenging old concepts. Clinical Microbiology Reviews. 18, (4): 719-756.
Pascucci, C. Cammà (2010). Spirochete della Malattia di Lyme nelle zecche raccolte in uno studio di campo nell'Italia centrale (Regione Marche). Veterinaria Italiana, Vol. 46 (2), 173-180.
Passamonti F., Veronesi F., Cappelli K., Capomaccio S., Coppola G., Marenzoni M. L., Piergili Fioretti D., Verini Supplizi A., Coletti M. (2010). Anaplasma phagocytophilum in horses and ticks: A preliminary survey of Central Italy. Comparative Immunology, Microbiology and Infectious Diseases, 33, (1): 73-83.
Petrovec M., Lotric Furlan S., Zupanc T. A., Strle F., Brouqui P., Roux V., Dumler J. S. (1997). Human disease in Europe caused by a granulocytic Ehrlichia species. Journal of Clinical Microbiology. Jun., 35(6): 1556-1559.
Punda-Polic V., Petrovec M., Trilar T., Duh D., Bradaric N., Klismanic Z., Avsic-Zupanc T. (2002). Detection and identification of spotted fever group rickettsiae in ticks collected in southern Croatia. Experimental and Applied Acarology. 28, (1-4): 169-176.
Roux V., Rydkina E., Eremeeva M., Raoult D. (1997) Citrate synthase gene comparison a new tool for phylogenetic analysis and its application for the Rickettsiae. International journal of systematic bacteriology. Vol. 47, (2): 252-261.
Ruscio M., Cinco M., (2003). Human granulocytic ehrlichiosis in Italy: first report on two confirmed cases. Annals of the New York Academy of Sciences. 990, 350-352.
Schwaiger M., Cassinotti P. (2003). Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick-borne encephalitis virus (TBEV) RNA. Journal of Clinical Virology, 27, (2): 136- 145.
Skotarczak B., Wodecka B., Cichocka A. (2002). Coexistence DNA of Borrelia burgdorferi sensu lato and Babesia microti in Ixodes ricinus ticks from north western Poland. Annals of Agricultural and Environmental Medicine. 9 (1): 25-28.
Veronesi F., Laus F., Passamonti F., Tesei B., Piergili Fioretti B., Genchi C. (2012). Occurrence of Borrelia lusitaniae infection in horses. Veterinary Microbiology. 160 (3-4): 535-538.

Costarelli 2017 et al., Infectious agents identified in ticks collected in Umbria region (Italy) - Agenti infettivi identificati in zecche raccolte nella regione Umbria (Italy). (SPVet.it 100/2017)









